chemical compound
Factsheet
Names IUPAC name
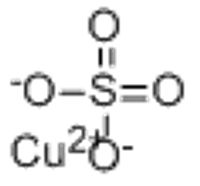
Copper(II) sulfate
Copper(II) sulfate
Identifiers
CAS Number 7758-98-7 (anhydrous) Y
7758-99-8 (pentahydrate) Y
16448-28-5 (trihydrate) N
19086-18-1 (heptahydrate) N
7758-99-8 (pentahydrate) Y
16448-28-5 (trihydrate) N
19086-18-1 (heptahydrate) N
Names IUPAC name
Copper(II) sulfate
Copper(II) sulfate
Identifiers
CAS Number 7758-98-7 (anhydrous) Y
7758-99-8 (pentahydrate) Y
16448-28-5 (trihydrate) N
19086-18-1 (heptahydrate) N
7758-99-8 (pentahydrate) Y
16448-28-5 (trihydrate) N
19086-18-1 (heptahydrate) N
Wikipedia
en.wikipedia.org › wiki › Copper(II)_sulfate
Copper(II) sulfate - Wikipedia
December 6, 2025 - Copper(II) sulfate is an inorganic compound with the chemical formula · CuSO4. It forms hydrates · CuSO4·nH2O, where n can range from 1 to 7. The pentahydrate (n = 5), a bright blue crystal, is the most commonly encountered hydrate of copper(II) sulfate, while its anhydrous form is white.
Videos
Molar Mass / Molecular Weight of CuSO4: Copper (II) sulfate - YouTube
01:56
What is the Molecular Formula or Molar Mass of Copper II Sulfate ...
01:33
How to Write the Formula for Copper (II) sulfate pentahydrate - ...
01:12
How to Write the Formula for Copper (II) sulfate - YouTube
02:07
How to Draw the Lewis Dot Structure for CuSO4: Copper (II) sulfate ...
01:43
How to Write the Name for CuSO4 - YouTube
RSC Education
edu.rsc.org › experiments › finding-the-formula-of-hydrated-copperii-sulfate › 436.article
Finding the formula of hydrated copper(II) sulfate | Class experiment | RSC Education
July 14, 2025 - In this experiment, a known mass of hydrated copper(II) sulfate is heated to remove the water of crystallisation · This is a class experiment suitable for students who already have a reasonable understanding of the mole concept. The mass of water is found by weighing before and after heating. This information is used to find x in the formula CuSO4.xH2O, using mole calculations
Soft Schools
softschools.com › formulas › chemistry › copper_ii_sulfate_formula › 653
Copper (II) Sulfate Formula
Copper sulfate, also known as bluestone or blue vitriol, is an inorganic salt used as dyeing agent, as catalyst in some organic reaction and mainly as a fungicide to treat fruits and vegetable diseases. Formula and structure: Copper (II) sulfate chemical formula is CuSO4 and the molar mass ...
EMBL-EBI
ebi.ac.uk › chebi › searchId.do
copper(II) sulfate (CHEBI:23414)
Chemical Entities of Biological Interest (ChEBI) is a freely available dictionary of molecular entities focused on 'small' chemical compounds.
ChemSpider
chemspider.com › Chemical-Structure.22870.html
Copper(II) sulfate | CuO4S
BASIC COPPER SULFATE · Blue Copper · Blue copper (VAN) Blue Copperas · Blue stone · Blue Vitriol · Copper · Copper (I) Selenide · copper (ii) sulfate · Copper (II) sulphate · Copper (II) Sulphate anhydrous · Copper monosulfate · Copper monosulfate anhydrous, Copper vitriol anhydrous ·
ScienceDirect
sciencedirect.com › topics › biochemistry-genetics-and-molecular-biology › copper-ii-sulfate
Copper(II) Sulfate - an overview | ScienceDirect Topics
Copper(II) sulfate is a chemical compound with the formula CuSO₄, known for its high water solubility of 143 g/L at 0°C.
Brainly
brainly.com › chemistry › college › what is the chemical formula for copper(ii) sulfate?
a. cuso\(_4\)
b. cu\(_2\)so
c. cus
d. cu\(_2\)s
[FREE] What is the chemical formula for copper(II) sulfate? A. CuSO_4 B. Cu_2SO C. CuS D. Cu_2S - brainly.com
The chemical formula for copper(II) sulfate is CuSO4. The chemical formula for copper(II) sulfate is CuSO4.
CK-12 Foundation
ck12.org › all subjects › chemistry › ternary ionic compounds - naming and formulas › what is the formula for copper (ii) sulfate?
Flexi answers - What is the formula for Copper (II) Sulfate? | CK-12 Foundation
September 11, 2025 - The chemical formula for Copper (II) Sulfate is CuSO4.
Top answer 1 of 3
8
Final Answer: The chemical formula for copper(II) sulfate is CuSO₄, which consists of copper, sulfur, and oxygen. Its commonly known hydrated form is CuSO₄·5H₂O, also called copper(II) sulfate pentahydrate, which appears as blue crystals. This compound has various applications in chemistry and agriculture.
; Explanation: The chemical formula for copper(II) sulfate is CuSO₄. This compound consists of one copper (Cu) atom, one sulfur (S) atom, and four oxygen (O) atoms. The parentheses in the name indicate that copper is in the +2 oxidation state, which means it has lost two electrons.
In addition to its anhydrous form (CuSO₄), copper(II) sulfate can also exist as a hydrate. The most common hydrate is copper(II) sulfate pentahydrate, which has five water molecules associated with each formula unit. Its full formula is written as CuSO₄·5H₂O, where the dot represents the association with water. This pentahydrate form appears as blue crystals and is often used in laboratories as a laboratory reagent and in educational demonstrations.
Copper(II) sulfate is widely found in nature and has been used historically for various applications, including agriculture and chemistry. Its uses extend to demonstrating crystallization processes in classrooms as well as in agriculture as a fungicide.
; Examples & Evidence: For example, CuSO₄·5H₂O can be dissolved in water to perform experiments involving chemical reactions, demonstrating properties of electrolytes, and studying crystallization processes. In agriculture, it is used to treat plants and soil.
Copper(II) sulfate is well-documented in chemistry textbooks and academic resources, detailing its chemical structure, properties, and uses.
2 of 3
8
Final Answer: A ; Explanation: Just took the test got a 100 ;
Brainly
brainly.com › chemistry › middle school › what is the ionic compound formula for copper(ii) sulfate?
[FREE] What is the ionic compound formula for copper(II) sulfate? - brainly.com
Copper(II) sulfate is a chemical compound with the formula CuSO₄. It is composed of two ions: the copper (II) ion, denoted as Cu²⁺, and the sulfate ion, which has the formula SO₄²⁻. In this ionic compound, because the copper ion has ...
ECHEMI
echemi.com › home › news › faq › what is the chemical formula for copper ii sulfate
What Is The Chemical Formula For Copper ii Sulfate - ECHEMI.com
June 24, 2022 - To express exactly what is the chemical formula for copper ii sulfate, requires a substance description, this compound has been cataloged as inorganic, by the absence of carbon, constituted by copper, sulfur, and oxygen, it can be generally found in the form called copper sulfate i, with formula, CuSO4, and another form as copper sulfate ii.
Pearson
pearson.com › channels › general-chemistry › asset › 4349505f › what-is-the-chemical-formula-for-copperii-sulfate-cuso-cuso-cus-cus
What is the chemical formula for copper(II) sulfate? CuSO CuSO Cu... | Study Prep in Pearson+
What is the chemical formula for copper(II) sulfate? CuSO CuSO CuS CuS
Quizlet
quizlet.com › science › chemistry
Write the formula for copper (II) sulfate pentahydrate. a) C | Quizlet
\mathrm { C } _ { 12 } \mathrm { H } _ { 22 } \mathrm { O } _ { 11 } + 12 \mathrm { O } _ { 2 } \rightarrow 12 \mathrm { CO } _ { 2 } + 11 \mathrm { H } _ { 2 } \mathrm { O }C12H22O11+12O2→12CO2+11H2O List all of the mole ratios that can be determined from this equation.