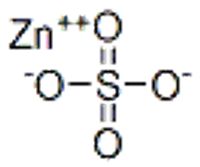chemical compound
Factsheet
Names IUPAC name
Zinc sulfate
White vitriol
Goslarite
Zinc sulfate
White vitriol
Goslarite
Identifiers
CAS Number 7733-02-0 Y
7446-19-7 (monohydrate) Y
13986-24-8 (hexahydrate) N
7446-20-0 (heptahydrate) Y
7446-19-7 (monohydrate) Y
13986-24-8 (hexahydrate) N
7446-20-0 (heptahydrate) Y
Names IUPAC name
Zinc sulfate
White vitriol
Goslarite
Zinc sulfate
White vitriol
Goslarite
Identifiers
CAS Number 7733-02-0 Y
7446-19-7 (monohydrate) Y
13986-24-8 (hexahydrate) N
7446-20-0 (heptahydrate) Y
7446-19-7 (monohydrate) Y
13986-24-8 (hexahydrate) N
7446-20-0 (heptahydrate) Y
Wikipedia
en.wikipedia.org › wiki › Zinc_sulfate
Zinc sulfate - Wikipedia
September 20, 2025 - Zinc sulfate is an inorganic compound with the formula ZnSO4. It forms hydrates ZnSO4·nH2O, where n can range from 0 to 7. All are colorless solids. The most common form includes water of crystallization as the heptahydrate, with the formula ZnSO4·7H2O.
Videos
00:51
Chemical formula of zinc sulphate l zinc sulphate formula l ZnSO4 ...
01:19
How to Write the Name for ZnSO4 - YouTube
01:06
How to Write the Formula for Zinc sulfate - YouTube
01:19
Molar Mass of ZnSO4: Zinc sulfate - YouTube
01:45
Is ZnSO4 acidic, basic, or neutral (dissolved in water)? - YouTube
What is zinc sulfate used for?
Zinc is a mineral that occurs naturally. Zinc is important for growth, and for body tissue development and health. Zinc sulfate is used to treat zinc shortages and avoid them.
byjus.com
byjus.com › chemistry › zinc-sulfate
Properties of Zinc Sulfate – ZnSO 4
What is the difference between zinc and zinc sulfate?
Zinc sulfate is a water-soluble form, meaning it is absorbed readily by the body. In OTC lozenges this chelated form is also used to avoid colds or to treat deficiencies in zinc. A further type of chelated zinc is zinc gluconate. This is commonly found in sprays of nasal zinc and is often called Orazinc.
byjus.com
byjus.com › chemistry › zinc-sulfate
Properties of Zinc Sulfate – ZnSO 4
Q1. What is Zinc Sulfate Good For?
Ans: Zinc is one of the naturally occurring minerals. Zinc plays an important role in the growth and development of healthy body tissues. Further, zinc sulfate can be utilized to treat and prevent diseases caused by zinc deficiency.
vedantu.com
vedantu.com › formula › zinc sulfate formula
Zinc Sulfate Formula - Definition, Uses, Toxicity and FAQs
CAMEO Chemicals
cameochemicals.noaa.gov › chemical › 4826
ZINC SULFATE | CAMEO Chemicals | NOAA
Anhydrous zinc sulfate is a colorless crystalline solid. Zinc sulfate is also obtained as a hexahydrate, ZnSO4.6H2O, and as a heptahydrate ZnSO4.7H2O. All forms are soluble in water. All are noncombustible. The primary hazard is the threat posed to the environment.
BYJUS
byjus.com › chemistry › zinc-sulfate
Properties of Zinc Sulfate – ZnSO 4
August 4, 2022 - Zinc sulfate is a water-soluble form, meaning it is absorbed readily by the body. In OTC lozenges this chelated form is also used to avoid colds or to treat deficiencies in zinc. A further type of chelated zinc is zinc gluconate. This is commonly found in sprays of nasal zinc and is often called Orazinc. Learn more about the physical and chemical properties, uses and structure of ZnSO4 ...
VEDANTU
vedantu.com › formula › zinc sulfate formula
Zinc Sulfate Formula - Definition, Uses, Toxicity and FAQs
June 24, 2021 - The Zinc sulphate ZnSo4 molecular weight will vary depending on its form. The molecular mass of zinc sulphate in anhydrous form is 161.47 g/mol, zinc sulphate in monohydrate form is 179.47 g/mol and zinc sulphate in heptahydrate form is 287.53 g/mol. Further, the density of zinc sulphate is about 3.54 g/cm3. The boiling point of zinc sulphate stays at 740 °C and further the melting point of the zinc sulfate chemical formula is 680 °C.
American Elements
americanelements.com › zinc-sulfate-7733-02-0
Zinc Sulfate | AMERICAN ELEMENTS ®
Linear Formula: ZnSO4 · MDL Number MFCD00011302 · EC No.: 231-793-3 · Zinc Sulfate is a moderately water and acid soluble Zinc source for uses compatible with sulfates. Sulfate compounds are salts or esters of sulfuric acid formed by replacing one or both of the hydrogens with a metal.
EMBL-EBI
ebi.ac.uk › chebi › searchId.do
zinc sulfate (CHEBI:35176)
Chemical Entities of Biological Interest (ChEBI) is a freely available dictionary of molecular entities focused on 'small' chemical compounds.
YouTube
youtube.com › watch
Equation for ZnSO4 + H2O (Zinc sulfate + Water) - YouTube
In this video we will describe the equation ZnSO4 + H2O and write what happens when ZnSO4 is dissolved in water.When ZnSO4 is dissolved in H2O (water) it wil...
Published June 29, 2020
WebQC
webqc.org › molecular-weight-of-znso4.html
ZnSO4 (Zinc sulfate) molar mass
Molar mass calculator computes molar mass, molecular weight and elemental composition of any given compound.
New Jersey Department of Health
nj.gov › health › eoh › rtkweb › documents › fs › 2044.pdf pdf
Right to Know Hazardous Substance Fact Sheet Common Name: ZINC SULFATE
Common Name: ZINC SULFATE · Synonyms: White Vitriol; Zinc Vitriol · CAS No: 7733-02-0 · Molecular Formula: ZnSO4 · RTK Substance No: 2044 · Description: Colorless, odorless, crystalline powder · HAZARD DATA · Hazard Rating · Firefighting · Reactivity ·
DrugBank
go.drugbank.com › drugs › DB09322
Zinc sulfate: Uses, Interactions, Mechanism of Action | DrugBank
Zinc sulfate is the inorganic compound with the formula ZnSO4 and historically known as "white vitriol".
DrugBank
go.drugbank.com › salts › DBSALT001304
Zinc sulfate monohydrate | DrugBank
Zinc sulfate is the inorganic compound with the formula ZnSO4 and historically known as "white vitriol".
Quora
quora.com › What-is-zinc-sulphate
What is zinc sulphate? - Quora
You can obtain this salt in various ways: Classic way → acid + base: H2SO4 + Zn(OH)2 → ZnSO4 + 2 H2O From Oxide → acid + basic oxide: → H2SO4 + ZnO → Zn SO4 + H2O From acid oxide (anhydride) + base: SO3 + Zn(OH)2 → ZnSO4 ...
NIST Chemistry WebBook
webbook.nist.gov › cgi › cbook.cgi
zinc sulphate
By formula: C4H10Zn (l) + (H2O4S • 100H2O) (solution) = 2C2H6 (g) + (O4SZn • 100H2O) (solution)