chemical compound
Factsheet
Names IUPAC name
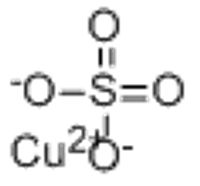
Copper(II) sulfate
Copper(II) sulfate
Identifiers
CAS Number 7758-98-7 (anhydrous) Y
7758-99-8 (pentahydrate) Y
16448-28-5 (trihydrate) N
19086-18-1 (heptahydrate) N
7758-99-8 (pentahydrate) Y
16448-28-5 (trihydrate) N
19086-18-1 (heptahydrate) N
Names IUPAC name
Copper(II) sulfate
Copper(II) sulfate
Identifiers
CAS Number 7758-98-7 (anhydrous) Y
7758-99-8 (pentahydrate) Y
16448-28-5 (trihydrate) N
19086-18-1 (heptahydrate) N
7758-99-8 (pentahydrate) Y
16448-28-5 (trihydrate) N
19086-18-1 (heptahydrate) N
Wikipedia
en.wikipedia.org › wiki › Copper(II)_sulfate
Copper(II) sulfate - Wikipedia
December 6, 2025 - Copper(II) sulfate pentahydrate decomposes before melting.
ScienceDirect
sciencedirect.com › topics › biochemistry-genetics-and-molecular-biology › copper-ii-sulfate
Copper(II) Sulfate - an overview | ScienceDirect Topics
The melting point is 1083°C with a boiling point of 2595°C. Copper has two stable isotopes, 63Cu and 65Cu, with natural abundances of 69.2 and 30.8%, respectively (Georgopoulos et al., 2001). The water solubility of copper (II) sulfate is 143 g/L at 0°C, whereas cuprous(I) oxide and copper ...
ChemicalBook
chemicalbook.com › ChemicalProductProperty_US_CB7751862.aspx
7758-98-7 CAS MSDS (Copper(II) sulfate) Melting Point Boiling Point Density CAS Chemical Properties
S. P. COPPER(II) SULFATE, REAGENTPLUS TM, >= 99%
Sciencemadness Wiki
sciencemadness.org › smwiki › index.php › Copper(II)_sulfate
Copper(II) sulfate - Sciencemadness Wiki
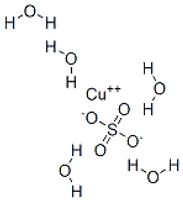
Copper sulfate pentahydrate occurs in nature as mineral chalcantite. ... Copper(II) sulfate is a blue crystalline solid as the pentahydrate, as it is most commonly seen, and the anhydrous form is a white to light gray powder. It has a solubility of 31.6 g/100 mL at 0˚C and 203.3 g/100 mL at 100˚C.
ChemEurope
chemeurope.com › en › encyclopedia › Copper(II)_sulfate.html
Copper(II)_sulfate
Copper(II) sulfate Copper(II) sulfate IUPAC name Copper(II) sulfatepentahydrate Other names Copper(II) sulfateCopper(II)sulphateCupric sulfateBlue
Chem-Impex
chemimpex.com › home › copper(ii) sulfate pentahydrate
Copper(II) sulfate pentahydrate – Chem-Impex
Copper(II) sulfate pentahydrate
Copper(II) sulfate pentahydrate is widely utilized in research focused on various applications:
Agriculture: This compound is commonly used as a fungicide and herbicide, helping to protect crops from fungal infections and pests, thus improving yield and quality.
Water Treatment: It serves as an algicide in water treatment facilities, effectively controlling algae growth in ponds and reservoirs, which helps maintain water quality.
Laboratory Reagent: In laboratories, it is employed as a reagent in various chemical reactions and experiments, particularly in analytical chemistry
Price $53.48
ChemicalBook
chemicalbook.com › ProductChemicalPropertiesCB7751862_EN.htm
Copper(II) sulfate CAS#: 7758-98-7
ChemicalBook provide Chemical industry users with Copper(II) sulfate Boiling point Melting point,Copper(II) sulfate Density MSDS Formula Use,If You also need to Copper(II) sulfate Other information,welcome to contact us.
Inchem
inchem.org › documents › icsc › icsc › eics1416.htm
ICSC 1416 - COPPER(II) SULFATE, PENTAHYDRATE
According to UN GHS Criteria · Transportation UN Classification
Sigma-Aldrich
sigmaaldrich.com › substance › copper(ii) sulfate
Copper(II) sulfate - Cupric sulfate
Melting Point (°C) Physical Form · Product Line · Purity · Quality Segment · Application · Markush Group · Reaction Type · Core · Special Grade · Puriss · Anhydrous · Puriss P.A. Filter Products · Showing 1-6 of 6 · Compare · Product Number · Description · Pricing · Copper(II) sulfate, ReagentPlus®, ≥99% Expand ·
American Elements
americanelements.com › copper-ii-sulfate-pentahydrate-7758-99-8
Copper(II) Sulfate Pentahydrate | AMERICAN ELEMENTS ®
Copper(II) Sulfate Pentahydrate is a moderately water and acid soluble Sodium source for uses compatible with sulfates. Sulfate compounds are salts or esters of sulfuric acid formed by replacing one or both of the hydrogens with a metal. Most metal sulfate compounds are readily soluble in water for uses such as water treatment, unlike fluorides and oxides which tend to be insoluble.
ChemSpider
chemspider.com › Chemical-Structure.22870.html
Copper(II) sulfate | CuO4S
BASIC COPPER SULFATE · Blue Copper · Blue copper (VAN) Blue Copperas · Blue stone · Blue Vitriol · Copper · Copper (I) Selenide · copper (ii) sulfate · Copper (II) sulphate · Copper (II) Sulphate anhydrous · Copper monosulfate · Copper monosulfate anhydrous, Copper vitriol anhydrous ·
MilliporeSigma
sigmaaldrich.com › US › en › sds › aldrich › 451657 pdf
Aldrich - 451657 Page 1 of 14
: Copper(II) sulfate · Product Number · : 451657 · Brand · : Aldrich · Index-No. : 029-004-00-0 · CAS-No. : 7758-98-7 · 1.2 · Relevant identified uses of the substance or mixture and uses advised against · Identified uses · : Laboratory chemicals, Synthesis of substances ·
Alfa Chemistry
alfa-chemistry.com › products › material & chemicals › metal & ceramic materials › copper(ii) sulfate pentahydrate
CAS 7758-99-8 Copper(II) sulfate pentahydrate - Alfa Chemistry
The melting point is 110 °C with decomposition. What is the odor of Copper(II) sulfate pentahydrate?
Sigma-Aldrich
sigmaaldrich.com › search › copper ii sulfate melting point
Copper ii sulfate melting point | Sigma-Aldrich
72 Copper(II) oxide granular for ... Molecular Formula: Ca(NO3)2 • 4H2O Molecular Weight: 236.2 CAS Number: 13477-34-4 Melting Point: approximately 560 °C1 Synonyms: lime nitrate; nitrocalcite This product is cell culture...
Chembk
chembk.com › en › chem › Copper(II) sulfate
Copper(II) sulfate
Name:Copper(II) sulfate,CAS:77... Copper(II) sulfate.Molecular Fomula:CuO4S,Molar Mass:159.61,Density:3.603g/mLat 25°C(lit.),Melting Point:200°C (dec.)(lit.),Boling Point:330°C at 760 mmHg,Solubility:203 g/L (20 ºC),Vapor Presure:7.3 mm Hg ( 25 °C),MSDS,Hazard,...
CAS Common Chemistry
commonchemistry.cas.org › detail
Copper sulfate pentahydrate - CAS Common Chemistry
Quickly confirm chemical names, CAS Registry Numbers®, structures or basic physical properties by searching compounds of general interest or leveraging an API connection.