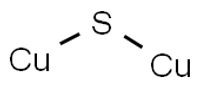
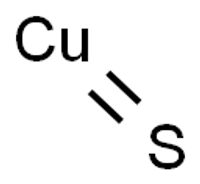chemical compound
Factsheet
Names IUPAC name
Copper(I) sulfide
Other names
Cuprous sulfide
Chalcocite
Copper glance
Copper(I) sulfide
Other names
Cuprous sulfide
Chalcocite
Copper glance
Identifiers
CAS Number 22205-45-4 Y
Names IUPAC name
Copper(I) sulfide
Other names
Cuprous sulfide
Chalcocite
Copper glance
Copper(I) sulfide
Other names
Cuprous sulfide
Chalcocite
Copper glance
Identifiers
CAS Number 22205-45-4 Y
Wikipedia
en.wikipedia.org › wiki › Copper(I)_sulfide
Copper(I) sulfide - Wikipedia
December 13, 2025 - Cu2O as an intermediate which is further reduced to the metal, and sulfur dioxide: ... Copper(I) oxide readily converts to copper(II) oxide when heated in the presence of oxygen, and to copper metal upon heating in a reducing environment.
American Elements
americanelements.com › copper-i-sulfide-22205-45-4
Copper(I) Sulfide | AMERICAN ELEMENTS ®
Copper(I) Sulfide is a crystalline solid used as a semiconductor and in photo optic applications.
Videos
01:31
How to Write the Formula for Copper (I) sulfide - YouTube
01:02
How to Write the Formula for Copper (II) sulfide - YouTube
01:27
How to Write the Name for Cu2S - YouTube
03:49
Which Is The Correct Formula For Copper(I) sulfide? - YouTube
03:16
Find the Percent Composition of Cu2S, copper (I) sulfide - YouTube
01:17
How to find the molar mass of CuS (Copper (II) Sulfide) - YouTube
Wikipedia
en.wikipedia.org › wiki › Copper_sulfide
Copper sulfide - Wikipedia
August 12, 2025 - Prominent copper sulfide minerals include Cu2S (chalcocite) and CuS (covellite). In the mining industry, the minerals bornite or chalcopyrite, which consist of mixed copper-iron sulfides, are often referred to as "copper sulfides". In chemistry, a "binary copper sulfide" is any binary chemical compound of the elements copper and sulfur.
ScienceDirect
sciencedirect.com › topics › earth-and-planetary-sciences › copper-sulfide
Copper Sulfide - an overview | ScienceDirect Topics
It is utilized in applications including optoelectronics and as a cathode material in lithium rechargeable batteries. AI generated definition based on: Materials Chemistry and Physics, 2005 ... Press Enter to select rating, 1 out of 3 starsPress Enter to select rating, 2 out of 3 starsPress ...
AZoM
azom.com › article.aspx
Copper Sulfide (CuS) Semiconductors
September 10, 2013 - Copper sulfide occurs naturally in nature as a mineral called covellite. It conducts electricity moderately.
Thermo Fisher
thermofisher.com › home › shop all products › chemicals › salts and inorganics › inorganic salts › transition metal salts › copper(i) sulfide, 99.5% (metals basis)
Copper(I) sulfide, 99.5% (metals basis) 250 g | Buy Online | Thermo Scientific Chemicals
Copper(I) sulfide, 99.5% (metals basis)
Copper(I) sulfide is used in luminous paints, electrodes and in solar cells. It is also used for grain mounts, XRD and in microprobe standards for the identification of unknown minerals. It acts as a precursor for the preparation of copper(I) oxide and copper metal. Further, it acts as a catalyst. T
Price $251.65
Taylor & Francis
taylorandfrancis.com › knowledge › Engineering_and_technology › Chemical_engineering › Copper_sulfide
Copper sulfide - Knowledge and References | Taylor & Francis
It is formed when active sulfur reacts with copper at high temperatures, and a black layer of copper sulfide can form on copper metal exposed to H2S.From: Environmentally Friendly and Biobased Lubricants [2016], Deposition of stoichiometry – tailored amorphous Cu-S thin films by MOCVD technique [2023], Environmental Chemistry [2022], Lubricant Additives [2017]
American Elements
americanelements.com › copper-ii-sulfide-1317-40-4
Copper(II) Sulfide | AMERICAN ELEMENTS ®
Copper Sulfide is a crystalline solid used as a semiconductor and in photo optic applications.
ScienceDirect
sciencedirect.com › science › article › abs › pii › S0022459616300718
Fabrication and applications of copper sulfide (CuS) nanostructures - ScienceDirect
March 2, 2016 - This review article presents different fabrication procedures (under the headlines of solvothermal routes, aerosol methods, solution methods and thermolysis), and applications (photocatalytic degradation, ablation of cancer cells, electrode material in lithium ion batteries and in gas sensing, organic solar cells, field emission properties, super capacitor applications, photoelectrochemical performance of QDSCs, photocatalytic reduction of organic pollutants, electrochemical bio sensing, enhanced PEC characteristics of pre-annealed CuS film electrodes) of copper sulfide (Covellite).
Loradchemical
loradchemical.com › products › copper(ii)-sulfide
Copper(II) Sulfide | CAS 1317-40-4 | Lorad Chemical Corporation
Copper(II) Sulfide powder is available as a 99.99 pure (metals basis) black powder in batch quantities for use as a high-energy density cathode material in an organic electrolyte Li-ion battery, as an electro-ceramic powder, or as a catalyst in other reactions.
WebElements
winter.group.shef.ac.uk › webelements › compounds › copper › copper_sulphide.html
WebElements Periodic Table » Copper » copper sulphide
This WebElements periodic table page contains copper sulphide for the element copper
Stanford Advanced Materials
samaterials.com › copper › 1846-copper-sulfide-cu2s-powder-chunk-lumps.html
Copper Sulfide (Cu2S) Powder/Chunk/Lumps (CAS No.22205-45-4) | Stanford Advanced Materials
It is a sulfide with an orthorhombic crystal system. The term chalcocite comes from the alteration of the obsolete name chalcosine, from the Greek khalkos, meaning copper. It is also known as redruthite, vitreous copper and copper-glance. ... Photovoltaic Cells: CuS is used in thin-film solar cells due to its semiconducting properties and ability to absorb sunlight effectively.
ESPI Metals
espimetals.com › index.php › msds › 545-Copper Sulfide CuS
Copper Sulfide CuS - ESPI Metals
Copper is mainly absorbed through the gastrointestinal tract. All other intakes of copper (inhalation and dermal) are insignificant in comparison to the oral route. Sulfides: Sulfides of the heavy metals are generally insoluble and hence have little toxic action except through the liberation of hydrogen sulfide.