chemical compound
Factsheet
Names IUPAC name
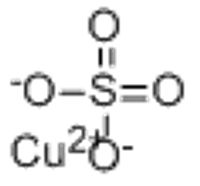
Copper(II) sulfate
Copper(II) sulfate
Identifiers
CAS Number 7758-98-7 (anhydrous) Y
7758-99-8 (pentahydrate) Y
16448-28-5 (trihydrate) N
19086-18-1 (heptahydrate) N
7758-99-8 (pentahydrate) Y
16448-28-5 (trihydrate) N
19086-18-1 (heptahydrate) N
Names IUPAC name
Copper(II) sulfate
Copper(II) sulfate
Identifiers
CAS Number 7758-98-7 (anhydrous) Y
7758-99-8 (pentahydrate) Y
16448-28-5 (trihydrate) N
19086-18-1 (heptahydrate) N
7758-99-8 (pentahydrate) Y
16448-28-5 (trihydrate) N
19086-18-1 (heptahydrate) N
Wikipedia
en.wikipedia.org › wiki › Copper(II)_sulfate
Copper(II) sulfate - Wikipedia
December 6, 2025 - Copper(II) sulfate is an inorganic compound with the chemical formula · CuSO4. It forms hydrates · CuSO4·nH2O, where n can range from 1 to 7. The pentahydrate (n = 5), a bright blue crystal, is the most commonly encountered hydrate of copper(II) sulfate, while its anhydrous form is white.
baseclick GmbH
baseclick.eu › home › products › copper(ii) sulfate (cuso4)
Copper(II) sulfate (CuSO4)
5 days ago - » Safety Data Sheet (PDF) Molecular Formula · CuSO4 · Shelf Life · 12 months unopened after receipt · Storage Conditions · RT, dry, inert gas · Molecular Weight · 159.6 g/mol · Purity · ≥ 98% (iodometric) Physical State · light green to grey fine powder · CAS Number · 7758-98-7 · Solubility · aqueous media · Preparation/Handling · Water-soluble copper(I) source for click reactions after reduction (e.g. by using BCMI-005) Copper(II) sulfate (CuSO₄) is a widely used copper source for copper-catalyzed azide–alkyne cycloaddition (CuAAC), ...
Videos
01:56
What is the Molecular Formula or Molar Mass of Copper II Sulfate ...
Molar Mass / Molecular Weight of CuSO4: Copper (II) sulfate - YouTube
01:12
How to Write the Formula for Copper (II) sulfate - YouTube
01:33
How to Write the Formula for Copper (II) sulfate pentahydrate - ...
02:07
How to Draw the Lewis Dot Structure for CuSO4: Copper (II) sulfate ...
01:43
How to Write the Name for CuSO4 - YouTube
Soft Schools
softschools.com › formulas › chemistry › copper_ii_sulfate_formula › 653
Copper (II) Sulfate Formula
Copper sulfate, also known as bluestone or blue vitriol, is an inorganic salt used as dyeing agent, as catalyst in some organic reaction and mainly as a fungicide to treat fruits and vegetable diseases. Formula and structure: Copper (II) sulfate chemical formula is CuSO4 and the molar mass ...
RSC Education
edu.rsc.org › experiments › finding-the-formula-of-hydrated-copperii-sulfate › 436.article
Finding the formula of hydrated copper(II) sulfate | Class experiment | RSC Education
July 14, 2025 - In this experiment, a known mass of hydrated copper(II) sulfate is heated to remove the water of crystallisation · This is a class experiment suitable for students who already have a reasonable understanding of the mole concept. The mass of water is found by weighing before and after heating. This information is used to find x in the formula CuSO4.xH2O, using mole calculations
EMBL-EBI
ebi.ac.uk › chebi › searchId.do
copper(II) sulfate (CHEBI:23414)
Chemical Entities of Biological Interest (ChEBI) is a freely available dictionary of molecular entities focused on 'small' chemical compounds.
ChemSpider
chemspider.com › Chemical-Structure.22870.html
Copper(II) sulfate | CuO4S
ChemSpider record containing structure, synonyms, properties, vendors and database links for Copper(II) sulfate, 7758-98-7, ARUVKPQLZAKDPS-UHFFFAOYSA-L
ScienceDirect
sciencedirect.com › topics › biochemistry-genetics-and-molecular-biology › copper-ii-sulfate
Copper(II) Sulfate - an overview | ScienceDirect Topics
Copper(II) sulfate is a chemical compound with the formula CuSO₄, known for its high water solubility of 143 g/L at 0°C.
Thermo Fisher
thermofisher.com › home › shop all products › chemicals › salts and inorganics › inorganic salts › transition metal salts › copper(ii) sulfate, anhydrous, 98%
Copper(II) sulfate, anhydrous, 98% 250 g | Buy Online | Thermo Scientific Chemicals
Copper(II) sulfate, anhydrous, 98%
Copper(II)sulfate is used in electroplating and in mining industries. It is used as a dehydrating agent for forming and manipulating acetal groups. In analytical chemistry, it is used in Fehling's solution and Benedict's solution to test for reducing sugars. Also, used to test protein in Biuret test
Price $65.65
Pearson
pearson.com › channels › general-chemistry › asset › 4349505f › what-is-the-chemical-formula-for-copperii-sulfate-cuso-cuso-cus-cus
What is the chemical formula for copper(II) sulfate? CuSO CuSO Cu... | Study Prep in Pearson+
What is the chemical formula for copper(II) sulfate? CuSO CuSO CuS CuS
Scribd
scribd.com › document › 368181770 › riah-kim-determine-the-formula-of-hydrated-copper-ii-sulfate-1
Riah Kim - Determine The Formula of Hydrated Copper II Sulfate 1 | PDF | Applied And Interdisciplinary Physics | Chemistry
This document describes a chemistry lab experiment to determine the formula of hydrated copper(II) sulfate. The student aims to find the number of moles of water in the hydrate CuSO4·xH2O by heating samples of varying mass to drive off water ...
Brainly
brainly.com › chemistry › middle school › what is the ionic compound formula for copper(ii) sulfate?
[FREE] What is the ionic compound formula for copper(II) sulfate? - brainly.com
The chemical formula of an ionic ... ion. Using these guidelines, the chemical formula of copper (II) sulfate can be written as CuSO4....
CK-12 Foundation
ck12.org › all subjects › chemistry › ternary ionic compounds - naming and formulas › what is the formula for copper (ii) sulfate?
Flexi answers - What is the formula for Copper (II) Sulfate? | CK-12 Foundation
September 11, 2025 - The chemical formula for Copper (II) Sulfate is CuSO4.
Home Science Tools
homesciencetools.com › special › shop all equipment & supplies › copper ii sulfate (cupric), 30 g, powder
Copper II Cupric Sulfate Powder | 30 g | HST
Pentahydrate form of copper II (cupric) sulfate powder. Copper sulfate is a great chemical for growing beautiful blue crystals. Find copper sulfate powder's density, common uses of copper sulfate, its formula, and more below!
Sigma-Aldrich
sigmaaldrich.com › US › en › product › aldrich › 451657
Copper(II) sulfate anhydrous, powder, = 99.99 trace metals 7758-98-7
Anhydrous form of copper sulfate occurs as mineral hydrocyanite. It exhibits a rhombic crystalline morphology. It decomposes to its oxide at temperatures above 600oC. Hydrate of CuSO4 is prepared by the addition of dilute sulfuric acid on copper (II) oxide or copper (II) carbonate, blue triclinic crystals of pentahydrate are formed upon crystallization.