PubChem
pubchem.ncbi.nlm.nih.gov › compound › Copper-Sulfate
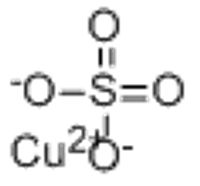
Copper Sulfate | CuSO4 | CID 24462 - PubChem
Copper Sulfate | CuSO4 or CuO4S | CID 24462 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.
chemical compound
Factsheet
Names IUPAC name
Copper(II) sulfate
Copper(II) sulfate
Identifiers
CAS Number 7758-98-7 (anhydrous) Y
7758-99-8 (pentahydrate) Y
16448-28-5 (trihydrate) N
19086-18-1 (heptahydrate) N
7758-99-8 (pentahydrate) Y
16448-28-5 (trihydrate) N
19086-18-1 (heptahydrate) N
Names IUPAC name
Copper(II) sulfate
Copper(II) sulfate
Identifiers
CAS Number 7758-98-7 (anhydrous) Y
7758-99-8 (pentahydrate) Y
16448-28-5 (trihydrate) N
19086-18-1 (heptahydrate) N
7758-99-8 (pentahydrate) Y
16448-28-5 (trihydrate) N
19086-18-1 (heptahydrate) N
Wikipedia
en.wikipedia.org › wiki › Copper(II)_sulfate
Copper(II) sulfate - Wikipedia
December 6, 2025 - Anhydrous copper sulfate is 39.81% copper and 60.19% sulfate by mass, and in its blue, hydrous form, it is 25.47% copper, 38.47% sulfate (12.82% sulfur) and 36.06% water by mass. Four types of crystal size are provided based on its usage: large crystals (10–40 mm), small crystals (2–10 ...
Videos
Molar Mass / Molecular Weight of CuSO4: Copper (II) sulfate - YouTube
01:10
Molar Mass / Molecular Weight of CuSO4 · 5H2O (Copper (II) Sulfate ...
01:56
What is the Molecular Formula or Molar Mass of Copper II Sulfate ...
01:36
molar mass of CuSO4 5H2O - YouTube
02:05
How to Find the Percent Composition by Mass for Copper (II) sulfate ...
WebQC
webqc.org › molecular-weight-of-copper(ii)sulfate.html
copper(ii)sulfate (CuSO4) molar mass
Molar mass calculator computes molar mass, molecular weight and elemental composition of any given compound.
WebQC
webqc.org › molecular-weight-of-cuso4.html
CuSO4 (Copper(II) sulfate) molar mass
Molar mass calculator computes molar mass, molecular weight and elemental composition of any given compound.
ITW Reagents
itwreagents.com › italy › en › product › l-0-1m-volumetric-solution › 181271
Copper(II) Sulfate 0.1 mol/l (0.1M) volumetric solution
The chemical formula CuSO4 stands for Copper(II) Sulfate. The molar mass of CuSO4 / Copper(II) Sulfate is 159,6 g/mol.
Quizlet
quizlet.com › science › chemistry
what is the molar mass of copper(ii) sulfate, cuso4? | Quizlet
\begin{matrix} \text{Temperature} & \text{Grams of KI per}\\ \ {\left({ }^{\circ} \mathrm{C}\right)} & \text{100.0 g Solution}\\ \text{20} & \text{144}\\ \text{40} & \text{162}\\ \text{60} & \text{176}\\ \text{80} & \text{192}\\ \text{100} & \text{206}\\ \end{matrix} Temperature (∘C)2040...
Homework.Study.com
homework.study.com › explanation › what-is-the-molar-mass-of-copper-ii-sulfate-cuso-4-a-16-00-g-mole-b-63-60-g-mole-c-111-6-g-mole-d-159-6-g-mole-e-319-2-g-mole.html
What is the molar mass of copper(II) sulfate, CuSO_4? A) 16.00 g/mole B) 63.60 g/mole C) 111.6 g/mole D) 159.6 g/mole E) 319.2 g/mole | Homework.Study.com
Answer to: What is the molar mass of copper(II) sulfate, CuSO_4? A) 16.00 g/mole B) 63.60 g/mole C) 111.6 g/mole D) 159.6 g/mole E) 319.2...
Brainly
brainly.com › chemistry › high school › what is the mass of one mole of copper(ii) sulfate (cuso\(_4\))?
[FREE] What is the mass of one mole of copper(II) sulfate (CuSO_4)? - brainly.com
Thus, the mass of one mole of copper(II) sulfate (CuSO₄) is 159.62 grams. For better understanding, consider other compounds like sodium chloride (NaCl) where the molar mass is 58.44 g/mol, calculated similarly by adding the molar masses of ...
Merck
merckmillipore.com › INTL › en › product › CopperII-sulfate-anhydrous,MDA_CHEM-102791
Copper(II) sulfate anhydrous CAS 7758-98-7 | 102791
Copper(II) sulfate anhydrous MSDS (material safety data sheet) or SDS, CoA and CoQ, dossiers, brochures and other available documents. ... CAS #: 7758-98-7 EC Number: 231-847-6 Molar Mass: 159.61 g/mol Chemical Formula: CuSO₄ Hill Formula: CuO₄S
Brainly
brainly.com › chemistry › college › what is the molar mass of copper(ii) sulfate, cuso\(_4\)?
a. 319.2 g/mole
b. 16.00 g/mole
c. 63.60 g/mole
d. 111.6 g/mole
e. 159.6 g/mole
[FREE] What is the molar mass of copper(II) sulfate, CuSO_4? A. 319.2 g/mole B. 16.00 g/mole C. 63.60 g/mole - brainly.com
To find the molar mass of copper(II) sulfate, CuSO4, we need to sum the molar masses of each element within the compound. The molar masses of copper (Cu), sulfur (S), and oxygen (O) are respectively 63.55 g/mol, 32.00 g/mol, and 16.00 g/mol ...
CK-12 Foundation
ck12.org › all subjects › chemistry › common ion effect › a chemist prepares a solution of copper(ii) sulfate (cus04) by measuring out 27.0 g of copper(ii) sulfate into a 500. ml volumetric flask and filling the flask to the mark with water. calculate the concentration in mol/l of the chemist's copper(ii) sulfate solution. round your answer to 2 significant digits.
Flexi answers - A chemist prepares a solution of copper(II) sulfate (CuS04) by measuring out 27.0 g of copper(II) sulfate into a 500. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/L of the chemist's copper(II) sulfate solution. Round your answer to 2 significant digits. | CK-12 Foundation
September 11, 2025 - First, we need to find the molar mass of copper(II) sulfate (CuSO4). The molar mass is calculated as follows: Cu: 63.55 g/mol S: 32.07 g/mol O: 16.00 g/mol × 4 = 64.00 g/mol Adding these together gives us a molar mass of 159.62 g/mol for CuSO4. Next, we convert the mass of CuSO4 to moles using ...
Vedantu
vedantu.com › question-answer › molar-mass-of-copperii-sulphate-class-12-chemistry-cbse-610cbe81c8186949fe45567b
What is the molar mass of copper(II) sulphate?
May 21, 2024 - Complete answer: The molar mass of a compound is often calculated by adding the quality atomic masses of the constituent atoms. -Make use of the chemical formula to determine the number of atoms of each element in the compound. -Multiply the atomic weight of each element with its number of atoms present in the compound.
Soft Schools
softschools.com › formulas › chemistry › copper_ii_sulfate_formula › 653
Copper (II) Sulfate Formula
Formula and structure: Copper (II) sulfate chemical formula is CuSO4 and the molar mass is 159.60 g mol-1. The compound is commonly found as a hydrated salt with between 1 to 5 molecules of water. The most common hydrated form is the CuSO4.5 H2O. The pentahydrated form has a molecular mass ...
ChemSpider
chemspider.com › Chemical-Structure.22870.html
Copper(II) sulfate | CuO4S
BASIC COPPER SULFATE · Blue Copper · Blue copper (VAN) Blue Copperas · Blue stone · Blue Vitriol · Copper · Copper (I) Selenide · copper (ii) sulfate · Copper (II) sulphate · Copper (II) Sulphate anhydrous · Copper monosulfate · Copper monosulfate anhydrous, Copper vitriol anhydrous ·
Unacademy
unacademy.com › question & answer › chemistry questions › calculate the formula mass of cuso4
Calculate the Formula Mass of CuSO4
June 8, 2022 - Multiply each atom’s atomic mass by the number of atoms in the compound. ... As a result, CuSO4 has a molar mass of 160 g/mol.
ChemicalAid
chemicalaid.com › molar mass calculator › cuso4(5h2o) (copper(ii) sulfate pentahydrate)
CuSO4(5H2O) Molar Mass
The molar mass and molecular weight of CuSO4(5H2O) / Copper(II) Sulfate Pentahydrate is 249.685.