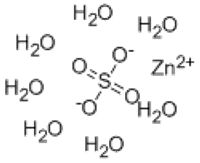
What are the component compounds of Zinc sulfate heptahydrate?
What is the CAS number of Zinc sulfate heptahydrate?
When was Zinc sulfate heptahydrate created and last modified?
OK so I am not a chemist and if these questions are too noobish just tell me to go away and read (and preferably what to read).
I wanted to order some Zinc Sulphate to combine with Sodium Bromide and boil it off to obtain Zinc Bromide for an electrolyte.
The only Zinc Sulphate I can find though is Zinc Sulphate Heptahydrate.
As I said I am not a chemist. I understand that the Heptahydrate bit means it has 7 molecules of water. Since water has a molar weight of 18.01528 g/mol and the Zinc Sulphate Heptahydrate has a molar weight of 287.46 g/mol does this mean that I just multiply the molar weight of the water by 7 and minus this from the total molar weight of the Zinc Sulphate Heptahydrate to get the molar weight of the Zinc Sulphate?
What happens to the water molecules when I make an aqueous solution do they just get released into the rest of water and I am left with a Zinc Sulphate solution? Should I use less water because of the extra water already included?