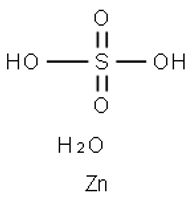
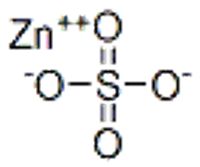chemical compound
Factsheet
Names IUPAC name
Zinc sulfate
White vitriol
Goslarite
Zinc sulfate
White vitriol
Goslarite
Identifiers
CAS Number 7733-02-0 Y
7446-19-7 (monohydrate) Y
13986-24-8 (hexahydrate) N
7446-20-0 (heptahydrate) Y
7446-19-7 (monohydrate) Y
13986-24-8 (hexahydrate) N
7446-20-0 (heptahydrate) Y
Names IUPAC name
Zinc sulfate
White vitriol
Goslarite
Zinc sulfate
White vitriol
Goslarite
Identifiers
CAS Number 7733-02-0 Y
7446-19-7 (monohydrate) Y
13986-24-8 (hexahydrate) N
7446-20-0 (heptahydrate) Y
7446-19-7 (monohydrate) Y
13986-24-8 (hexahydrate) N
7446-20-0 (heptahydrate) Y
Wikipedia
en.wikipedia.org › wiki › Zinc_sulfate
Zinc sulfate - Wikipedia
September 20, 2025 - When heated above 680 °C, zinc ... with sulfate and one water of crystallization by hydrogen bonds. Anhydrous zinc sulfate is isomorphous with anhydrous copper(II) sulfate....
Videos
zinc sulphate fertilizer | zinc sulphate monohydrate 33 | zinc33 ...
02:14
Zinc Sulphate Monohydrate (33% Zn) - Hindi - YouTube
JAM GROUP Mineral Processing on Instagram: "Zinc sulfate is ...
01:47
Equation for ZnSO4 + H2O (Zinc sulfate + Water) - YouTube
02:03
Molar Mass / Molecular Weight of ZnSO4 • 7H2O: Zinc sulfate ...
CAMEO Chemicals
cameochemicals.noaa.gov › chemical › 4826
ZINC SULFATE | CAMEO Chemicals | NOAA
Anhydrous zinc sulfate is a colorless crystalline solid. Zinc sulfate is also obtained as a hexahydrate, ZnSO4.6H2O, and as a heptahydrate ZnSO4.7H2O. All forms are soluble in water. All are noncombustible. The primary hazard is the threat posed to the environment.
The Good Scents Company
thegoodscentscompany.com › data › rw1100251.html
zinc sulfate anhydrous, 7733-02-0
Functional use(s) - cosmetic agents
GSRS
precision.fda.gov › ginas › app › ui › substances › deede46f-47fb-4e72-9be8-16a1c27410ac
ZINC SULFATE ANHYDROUS
A community for NGS assay evaluation
Sigma-Aldrich
sigmaaldrich.com › search › anhydrous zinc sulphate
Anhydrous zinc sulphate | Sigma-Aldrich
Find anhydrous zinc sulphate and related products for scientific research at MilliporeSigma
GSRS
gsrs.ncats.nih.gov › ginas › app › beta › substances › 0J6Z13X3WO
ZINC SULFATE ANHYDROUS
We cannot provide a description for this page right now
DrugBank
go.drugbank.com › drugs › DB09322
Zinc sulfate: Uses, Interactions, Mechanism of Action | DrugBank
This medication is a mineral used to treat or prevent low levels of zinc alone and together with oral rehydration therapy (ORT). It is also used as a topical astringent. Zinc Sulfate Injection, USP is indicated for use as a supplement to intravenous solutions given for TPN.
Patsnap Synapse
synapse.patsnap.com › article › what-is-the-mechanism-of-zinc-sulfate-hydrate
What is the mechanism of Zinc Sulfate Hydrate?
The effectiveness of these applications hinges on the compound's ability to dissociate and interact with other substances. Temperature and pH can influence the dissociation and stability of zinc sulfate hydrate. For example, heating can drive off the water of hydration, converting it to anhydrous zinc sulfate (ZnSO₄).
American Elements
americanelements.com › zinc-sulfate-7733-02-0
Zinc Sulfate | AMERICAN ELEMENTS ®
Zinc Sulfate is a moderately water and acid soluble Zinc source for uses compatible with sulfates. Sulfate compounds are salts or esters of sulfuric acid formed by replacing one or both of the hydrogens with a metal. Most metal sulfate compounds are readily soluble in water for uses such as ...
ChemSpider
chemspider.com › Chemical-Structure.22833.html
Zinc sulfate | O4SZn
Zinc sulfate (anhydrous) Zinc sulfate, anhydrous · Zinc Sulfate,Anhydrous · ZINC SULFATEANHYDROUS · Zinc sulfic acid · Zinc sulphate · Zinc sulphate (1:1) Zinc sulphate anhydrous · Zinc sulphate, anhydrous · Zinc sulphic acid · Zinc vitriol · Zinc vitriol (VAN) zinc(2+) ion sulfate ·
Inchem
inchem.org › documents › icsc › icsc › eics1698.htm
ICSC 1698 - ZINC SULFATE
According to UN GHS Criteria · Transportation UN Classification UN Hazard Class: 9; UN Pack Group: III
Sciencemadness Wiki
sciencemadness.org › smwiki › index.php › Zinc_sulfate
Zinc sulfate - Sciencemadness Wiki
Zinc sulfate has low toxicity and is also sold as a supplement. It's best to avoid consuming lab-grade ZnSO4 though, as it may contain traces of heavy metals (cadmium e.g.). Anhydrous zinc sulfate may cause irritations.
USDA
ams.usda.gov › sites › default › files › media › Zinc Sulfate report 2015.pdf pdf
Zinc Sulfate Livestock ___________________________________ February 3, 2015
There are four hydration states associated with zinc sulfate. A summary of their important physical ... properties is provided in Table 2. Anhydrous zinc sulfate is not found naturally.
Dynamicbiotech
dynamicbiotech.com › news › difference-between-zinc-sulphate-monohydrate-and-zinc.html
Difference Between Zinc Sulphate and Zinc Sulphate Monohydrate
Anhydrous zinc sulfate, being an anhydrous form, is relatively stable and less prone to absorbing moisture from the surrounding environment. It has low hygroscopicity, meaning it does not readily attract and retain water molecules.
Brenntag
brenntag.com › home › products › zinc sulphate
Zinc Sulphate Bulk Distributor | CAS 7733-02-0 (anhydrous), 7446-19-7 (monohydrate), 13986-24-8 (Hexahydrate), 7446-20-0 (heptahydrate) | Brenntag | Brenntag
zinc(2+) sulfate · Chemical Formula · ZnSO4 * H2O · CAS Number · 7733-02-0 (anhydrous), 7446-19-7 (monohydrate), 13986-24-8 (Hexahydrate), 7446-20-0 (heptahydrate) Molar Weight · 179.47 g/mol (monohydrate)|287.53 g/mol (heptahydrate) Melting Point · 100°C (heptahydrate), 70°C (hexahydrate, ...
Call +57 1 651 36 00
Address Carrera 15 No. 93a-84, oficina 606 T, Bogotá
NCATS Inxight Drugs
drugs.ncats.io › drug › 0J6Z13X3WO
ZINC SULFATE ANHYDROUS - Inxight Drugs
The National Center for Advancing Translational Sciences has developed Inxight Drugs — a comprehensive portal for drug development information. NCATS Inxight incorporates and unifies a wealth of data, including marketing and regulatory status, rigorous drug ingredient definitions, biological ...